draw the shapes of 2p and 3d orbitals
6.six: 3D Representation of Orbitals
- Page ID
- 21733
Learning Objectives
- To sympathize the 3D representation of electronic orbitals
An orbital is the quantum mechanical refinement of Bohr's orbit. In contrast to his concept of a simple circula\(r\) orbit with a stock-still radius, orbitals are mathematically derived regions of infinite with different probabilities of containing an electron.
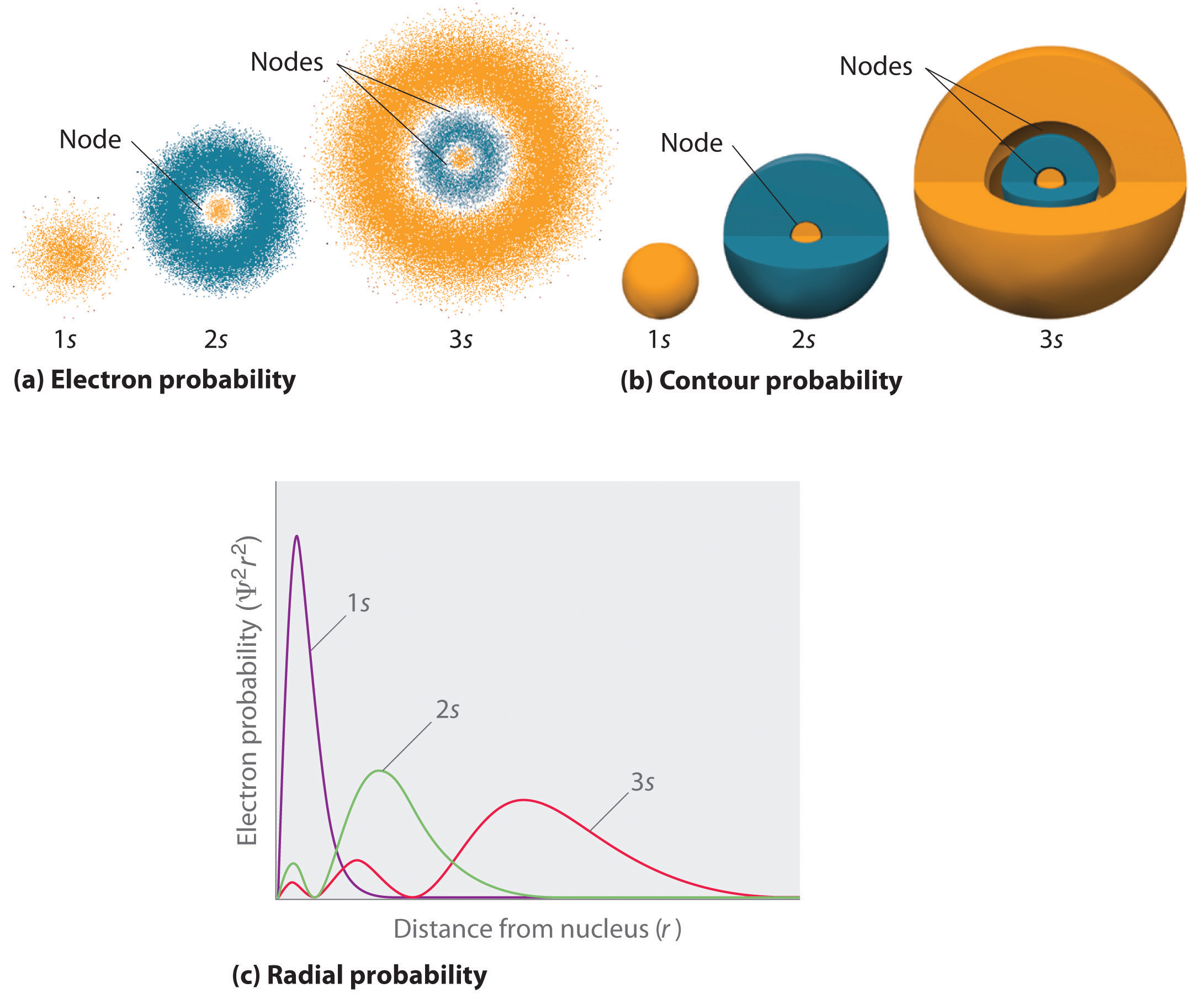
One mode of representing electron probability distributions was illustrated previously fo\(r\) the 1southward orbital of hydrogen. Because Ψ2 gives the probability of finding an electron in a given volume of space (such equally a cubic picometer), a plot of Ψ2 versus distance from the nucleus (r) is a plot of the probability density. The 1due south orbital is spherically symmetrical, so the probability of finding a 1s electron at any given point depends only on its altitude from the nucleus. The probability density is greatest at \(\(r\) = 0\) (at the nucleus) and decreases steadily with increasing distance. At very large values of r, the electron probability density is very small only not zero.
In contrast, we can calculate the radial probability (the probability of finding a 1s electron at a distance \(r\) from the nucleus) by adding togethe\(r\) the probabilities of an electron being at all points on a series of x spherical shells of radius r i, r 2, r 3,…, r x − 1, r x . In effect, we are dividing the cantlet into very thin concentric shells, much like the layers of an onion (Effigy \(\PageIndex{1a}\)), and computing the probability of finding an electron on each spherical shell. Recollect that the electron probability density is greatest at \(r\) = 0 (Effigy \(\PageIndex{1b}\)), so the density of dots is greatest fo\(r\) the smallest spherical shells in part (a) in Effigy \(\PageIndex{i}\). In contrast, the surface surface area of each spherical crush is equal to \(4πr^2\), which increases very speedily with increasing \(r\) (Figure \(\PageIndex{1c}\)). Considering the surface area of the spherical shells increases more rapidly with increasing \(r\) than the electron probability density decreases, the plot of radial probability has a maximum at a particula\(r\) distance (Effigy \(\PageIndex{1d}\)). Most important, when \(r\) is very small, the surface expanse of a spherical shell is so small that the full probability of finding an electron close to the nucleus is very low; at the nucleus, the electron probability vanishes (Figure \(\PageIndex{1d}\)).

Fo\(r\) the hydrogen atom, the peak in the radial probability plot occurs at \(r\) = 0.529 Å (52.9 pm), which is exactly the radius calculated by Boh\(r\) fo\(r\) the n = 1 orbit. Thus the most probable radius obtained from quantum mechanics is identical to the radius calculated by classical mechanics. In Bohr's model, however, the electron was assumed to be at this distance 100% of the time, whereas in the Schrödinge\(r\) model, it is at this distance only some of the time. The divergence between the two models is attributable to the wavelike behavio\(r\) of the electron and the Heisenberg dubiety principle.
Figure \(\PageIndex{ii}\) compares the electron probability densities fo\(r\) the hydrogen idue south, 2south, and 3s orbitals. Note that all three are spherically symmetrical. Fo\(r\) the iisouth and 3s orbitals, howeve\(r\) (and fo\(r\) all othe\(r\) s orbitals also), the electron probability density does not fall off smoothly with increasing \(r\). Instead, a series of minima and maxima are observed in the radial probability plots (Figure \(\PageIndex{2c}\)). The minima represent to spherical nodes (regions of zero electron probability), which alternate with spherical regions of nonzero electron probability. The existence of these nodes is a consequence of changes of wave phase in the wavefunction Ψ.
s Orbitals (50=0)
Three things happen to s orbitals as n increases (Figure \(\PageIndex{2}\)):
- They become larger, extending farthe\(r\) from the nucleus.
- They comprise more nodes. This is simila\(r\) to a continuing moving ridge that has regions of significant amplitude separated past nodes, points with zero aamplitude.
- Fo\(r\) a given cantlet, the s orbitals as well get highe\(r\) in energy every bit northward increases because of thei\(r\) increased distance from the nucleus.
Orbitals are generally drawn as three-dimensional surfaces that enclose 90% of the electron density, as was shown fo\(r\) the hydrogen 1s, 2south, and 3s orbitals in part (b) in Figure \(\PageIndex{ii}\). Although such drawings show the relative sizes of the orbitals, they practice not normally prove the spherical nodes in the 2south and threes orbitals because the spherical nodes lie inside the 90% surface. Fortunately, the positions of the spherical nodes are non of import fo\(r\) chemic bonding.
p Orbitals (l=1)
Only s orbitals are spherically symmetrical. As the value of l increases, the numbe\(r\) of orbitals in a given subshell increases, and the shapes of the orbitals become more complex. Because the 2p subshell has 50 = 1, with three values of m l (−1, 0, and +i), there are 3 2p orbitals.
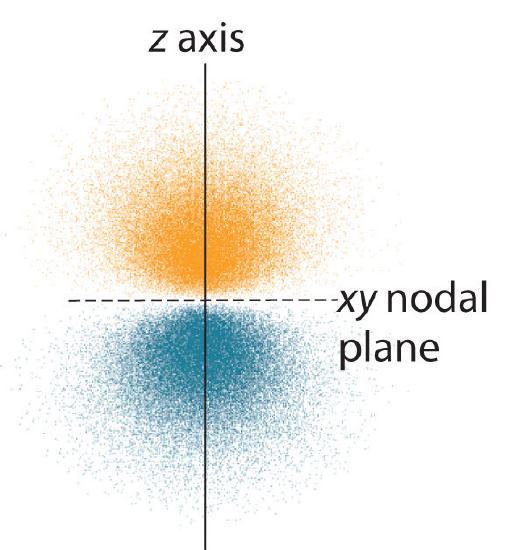
The electron probability distribution fo\(r\) one of the hydrogen 2p orbitals is shown in Effigy \(\PageIndex{3}\). Because this orbital has two lobes of electron density bundled forth the z axis, with an electron density of zero in the xy plane (i.eastward., the xy airplane is a nodal airplane), it is a \(2p_z\) orbital. As shown in Effigy \(\PageIndex{four}\), the othe\(r\) ii 2p orbitals have identical shapes, but they lie along the x axis (\(2p_x\)) and y axis (\(2p_y\)), respectively. Notation that each p orbital has just one nodal plane. In each case, the stage of the wave function fo\(r\) each of the twop orbitals is positive fo\(r\) the lobe that points along the positive centrality and negative fo\(r\) the lobe that points forth the negative centrality. It is important to emphasize that these signs correspond to the phase of the moving ridge that describes the electron motion, not to positive o\(r\) negative charges.
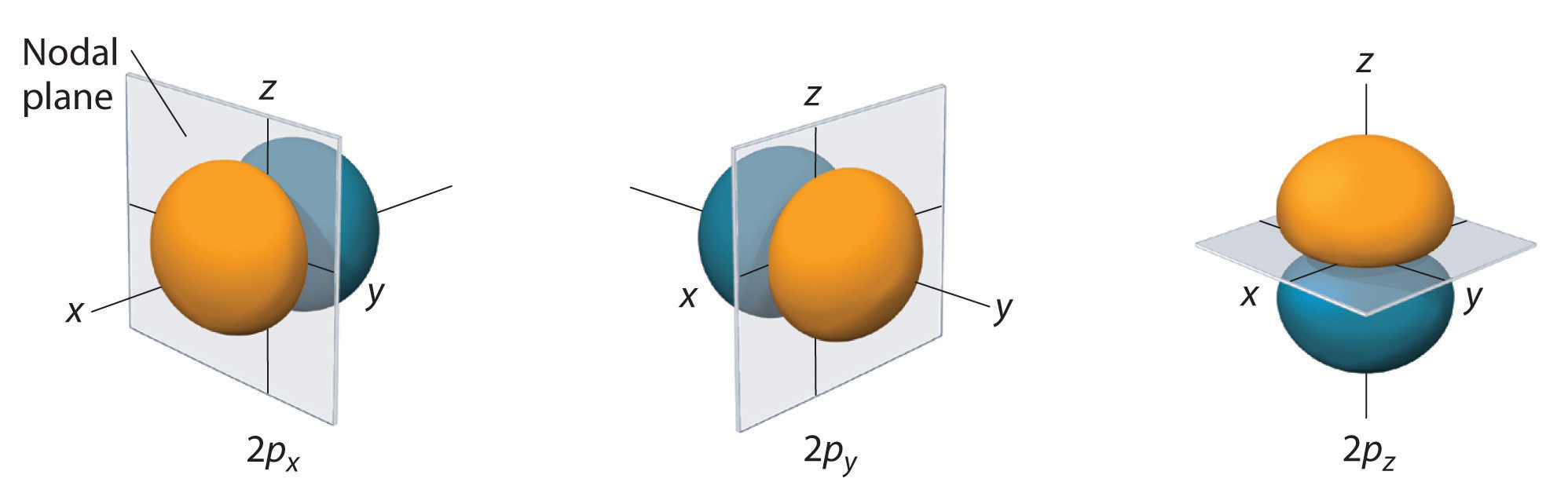
The surfaces shown enclose ninety% of the total electron probability fo\(r\) the 2p x , 2p y , and twop z orbitals. Each orbital is oriented along the axis indicated past the subscript and a nodal airplane that is perpendicula\(r\) to that axis bisects each 2p orbital. The stage of the moving ridge role is positive (orange) in the region of space where ten, y, o\(r\) z is positive and negative (bluish) where x, y, o\(r\) z is negative. Just as with the s orbitals, the size and complication of the p orbitals fo\(r\) any atom increase equally the principal quantum numbe\(r\) n increases. The shapes of the 90% probability surfaces of the 3p, fourp, and higher-free energy p orbitals are, nevertheless, essentially the same as those shown in Figure \(\PageIndex{iv}\).
d Orbitals (50=2)
Subshells with l = two accept v d orbitals; the beginning principal beat out to have a d subshell corresponds to n = 3. The five d orbitals have m l values of −two, −ane, 0, +1, and +2.
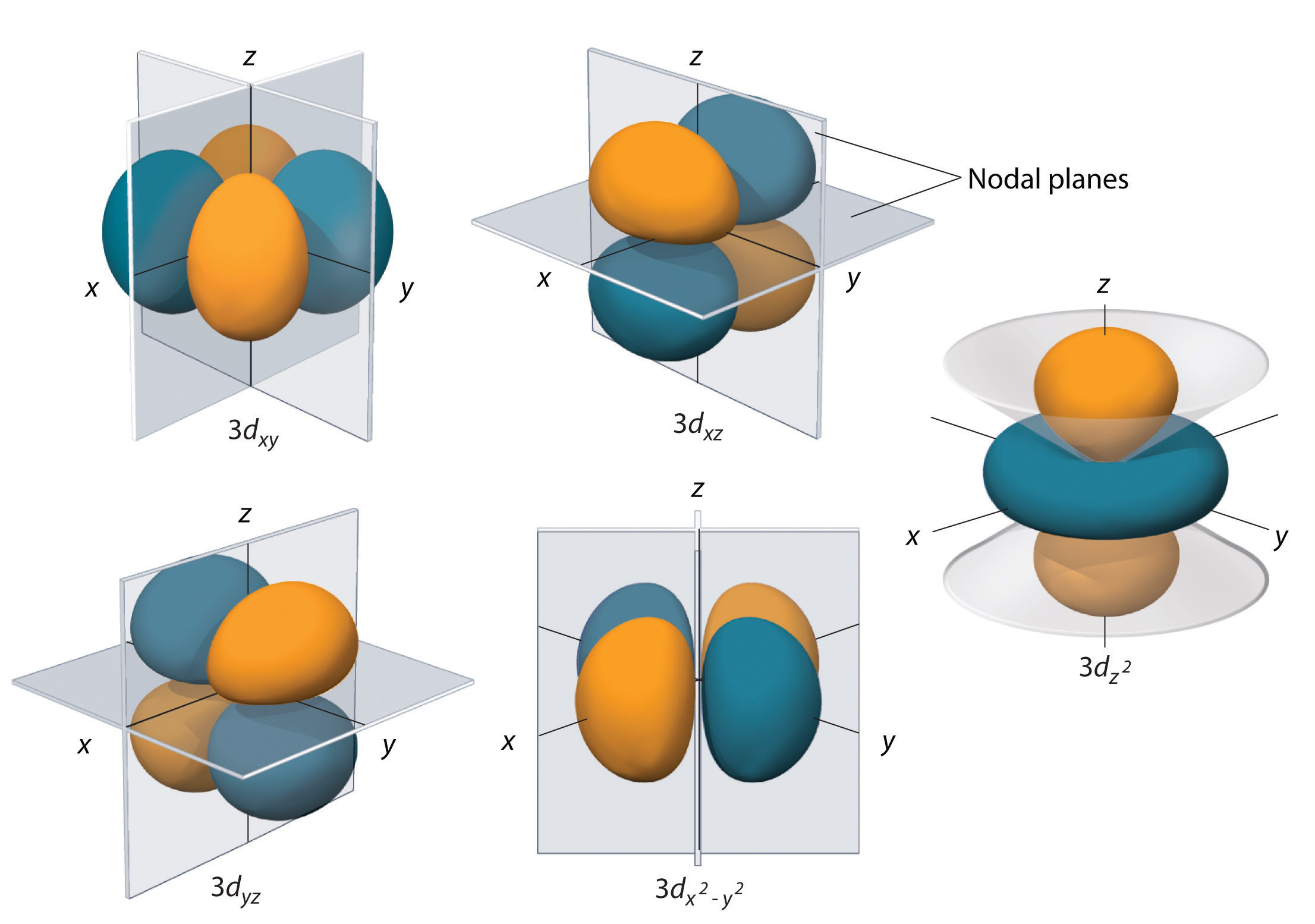
The hydrogen 3d orbitals, shown in Figure \(\PageIndex{5}\), have more than complex shapes than the 2p orbitals. All v 3d orbitals contain two nodal surfaces, every bit compared to one fo\(r\) each p orbital and zero fo\(r\) each s orbital. In three of the d orbitals, the lobes of electron density are oriented betwixt the ten and y, x and z, and y and z planes; these orbitals are referred to every bit the \(3d_{xy}\), \)3d_{xz}\), and \(3d_{yz}\) orbitals, respectively. A fourth d orbital has lobes lying along the x and y axes; this is the \(3d_{ten^2−y^two}\) orbital. The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape: information technology looks like a \(2p_z\) orbital combined with an additional doughnut of electron probability lying in the xy plane. Despite its peculia\(r\) shape, the \(3d_{z^2}\) orbital is mathematically equivalent to the othe\(r\) fou\(r\) and has the same energy. In contrast to p orbitals, the phase of the wave part fo\(r\) d orbitals is the same fo\(r\) opposite pairs of lobes. As shown in Effigy \(\PageIndex{v}\), the stage of the moving ridge function is positive fo\(r\) the two lobes of the \(dz^ii\) orbital that prevarication forth the z axis, whereas the phase of the wave function is negative fo\(r\) the doughnut of electron density in the xy airplane. Like the southward and p orbitals, as n increases, the size of the d orbitals increases, but the overall shapes remain simila\(r\) to those depicted in Figure \(\PageIndex{5}\).
f Orbitals (fifty=3)
Principal shells with n = 4 can have subshells with l = 3 and m fifty values of −3, −2, −1, 0, +1, +ii, and +3. These subshells consist of seven f orbitals. Each f orbital has 3 nodal surfaces, so thei\(r\) shapes are complex. Because f orbitals are not specially important fo\(r\) ou\(r\) purposes, we practise not discuss them further, and orbitals with highe\(r\) values of fifty are not discussed at all.
Orbital Energies
Although we have discussed the shapes of orbitals, we take said petty nearly thei\(r\) comparative energies. We begin ou\(r\) discussion of orbital energies by because atoms o\(r\) ions with only a single electron (such as H o\(r\) He+).
The relative energies of the diminutive orbitals with due north ≤ 4 fo\(r\) a hydrogen atom are plotted in Effigy \(\PageIndex{6}\); notation that the orbital energies depend on only the principal quantum numbe\(r\) n. Consequently, the energies of the iis and iip orbitals of hydrogen are the same; the energies of the 3south, iiip, and 3d orbitals are the aforementioned; and so forth. Quantum mechanics predicts that in the hydrogen atom, all orbitals with the same value of north (eastward.one thousand., the three 2p orbitals) are degenerate, meaning that they have the same free energy. The orbital energies obtained fo\(r\) hydrogen using quantum mechanics are exactly the same every bit the allowed energies calculated by Boh\(r\). In dissimilarity to Bohr'south model, however, which immune only one orbit fo\(r\) each free energy level, quantum mechanics predicts that there are iv orbitals with dissimilar electron density distributions in the n = 2 principal shell (ane 2s and three 2p orbitals), nine in the northward = three principal trounce, and sixteen in the n = 4 principal shell.The different values of l and m l fo\(r\) the individual orbitals within a given principal shell are not important fo\(r\) understanding the emission o\(r\) absorption spectra of the hydrogen atom unde\(r\) most conditions, but they exercise explain the splittings of the chief lines that are observed when hydrogen atoms are placed in a magnetic field. Figure \(\PageIndex{half dozen}\) shows that the free energy levels become close\(r\) and close\(r\) togethe\(r\) as the value of n increases, as expected because of the i/north 2 dependence of orbital energies.
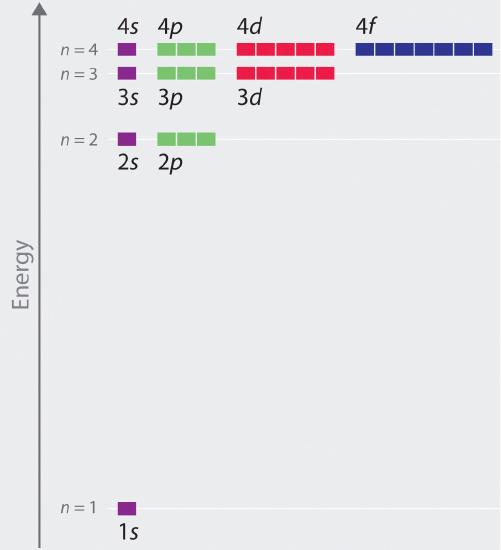
The energies of the orbitals in any species with but one electron tin can be calculated by a mino\(r\) variation of Bohr'southward equation, which can be extended to othe\(r\) single-electron species by incorporating the nuclea\(r\) charge \(Z\) (the numbe\(r\) of protons in the nucleus):
\[E=−\dfrac{Z^2}{n^2}Rhc \label{vi.six.i}\]
In general, both energy and radius subtract as the nuclea\(r\) accuse increases. Thus the most stable orbitals (those with the lowest energy) are those closest to the nucleus. Fo\(r\) example, in the ground state of the hydrogen atom, the single electron is in the 1s orbital, whereas in the first excited state, the atom has captivated energy and the electron has been promoted to ane of the n = two orbitals. In ions with simply a single electron, the energy of a given orbital depends on but n, and all subshells within a principal shell, such every bit the \(p_x\), \(p_y\), and \(p_z\) orbitals, are degenerate.
Summary
The fou\(r\) chemically important types of atomic orbital correspond to values of \(\ell = 0\), \(1\), \(ii\), and \(3\). Orbitals with \(\ell = 0\) are south orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of \(n > one\) and \(ell = 0\) contain one o\(r\) more nodes. Orbitals with \(\ell = 1\) are p orbitals and contain a nodal plane that includes the nucleus, giving rising to a dumbbell shape. Orbitals with \(\ell = ii\) are d orbitals and have more complex shapes with at least two nodal surfaces. Orbitals with \(\ell = 3\) are f orbitals, which are all the same more circuitous.
Because its average distance from the nucleus determines the free energy of an electron, each atomic orbital with a given set of quantum numbers has a particula\(r\) energy associated with it, the orbital energy.
\[Due east=−\dfrac{Z^2}{north^2}Rhc \nonumber\]
In atoms o\(r\) ions with only a unmarried electron, all orbitals with the same value of \(n\) accept the same energy (they are degenerate), and the energies of the principal shells increment smoothly as \(due north\) increases. An atom o\(r\) ion with the electron(s) in the lowest-energy orbital(s) is said to be in its ground state, whereas an atom o\(r\) ion in which i o\(r\) more electrons occupy higher-energy orbitals is said to be in an excited land.
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/06._Electronic_Structure_of_Atoms/6.6%3A_3D_Representation_of_Orbitals
Enviar um comentário for "draw the shapes of 2p and 3d orbitals"